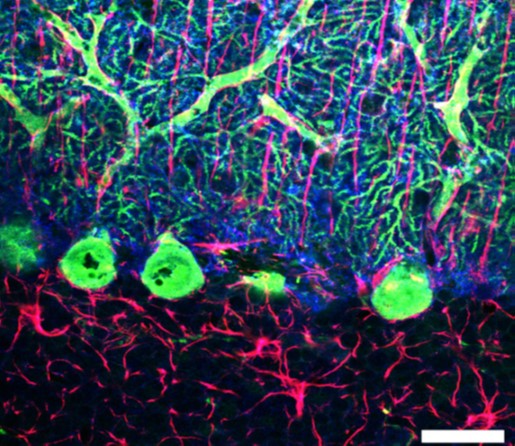
Triple labeling confocal microscopy (GFAP–red, calbindin– green, GLAST–blue) in the cerebellar cortex of ataxic mice in the early phase of disease. Scale bar: 20μm.
Mitochondrial transfer and tracking
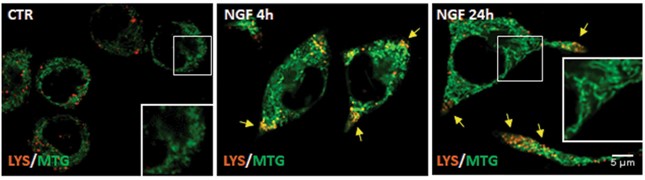 Representative images of PC12-615 cells treated with NGF (10 ng/ml) for 4–24 h, followed by addition of Lysotracker-red and Mitotracker-green during the last 30 min of incubation. Scale bar 5 μm.
Representative images of PC12-615 cells treated with NGF (10 ng/ml) for 4–24 h, followed by addition of Lysotracker-red and Mitotracker-green during the last 30 min of incubation. Scale bar 5 μm.
Martorana F, Gaglio D, Bianco MR, Aprea F, Virtuoso A, Bonanomi M, Alberghina L, Papa M, Colangelo AM. Differentiation by nerve growth factor (NGF) involves mechanisms of crosstalk between energy homeostasis and mitochondrial remodeling. Cell Death Dis. 2018 Mar 9;9(3):391. doi: 10.1038/s41419-018-0429-9. PMID: 29523844; PMCID: PMC5844953.
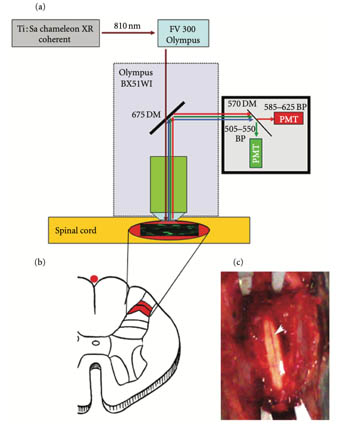
2P in vivo microscopy of spinal cord
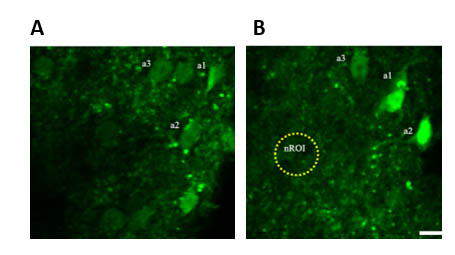
Sensory stimulation triggers Ca2+ increase in spinal astrocytes. Section of superficial laminae of dorsal horn of lumbar spinal cord, showing astrocytes (a1-3) in rest condition (A) and following sensory stimulation (B) (scale bar: 10 μm).
a) Two-photon laser scanning microscope setup. (b) Schematic section of the lumbar spinal cord showing the regions of interest (laminae I-II). (c) Exposed posterior spinal cord surface (in the middle line, the posterior medial spinal vein).
Neural tissue engineering techniques
3D silk fibroin engraftment in rat brain. Triple labeling confocal microscopy (silk fibroin engraftment-red; mesenchymal stem cells-green; DAPI nuclear staining-blue).
Scale bar: 500 μm
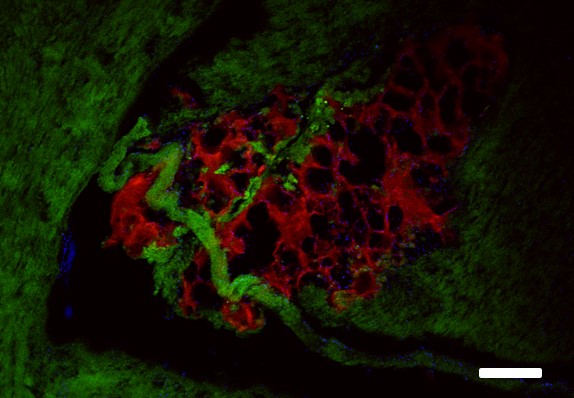
Glio-vascular coupling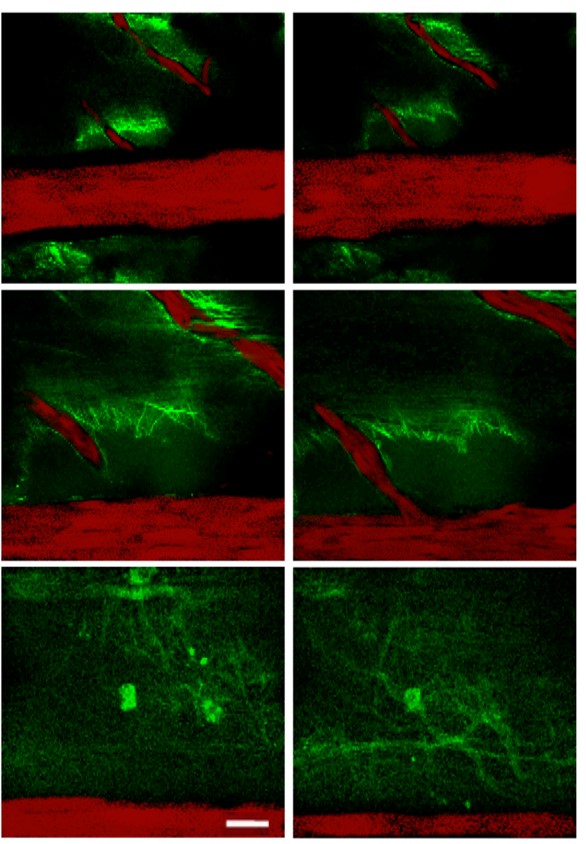
In vivo Calcium imaging (2-photon microscopy)
Time-lapse/z stack serial images of lumbar spinal cord. Spinal vessels are labeled with Dextran-Rhodamine; astrocytes adjacent to spinal vessels are labeled with OGB (scale bar: 10 μm).
Cirillo, G., De Luca, D., Papa, M., 2012. Calcium imaging of living astrocytes in the mouse spinal cord following sensory stimulation. Neural Plast 2012, 425818. https://doi.org/10.1155/2012/425818
Functional magnetic resonance imaging (fMRI)
fMRI
Functional magnetic resonance imaging (fMRI) is an advanced imaging technique for qualitative and quantitative evaluation of brain activity. The procedure is similar to conventional, structural MRI but uses the change in magnetization between oxygen-rich and oxygen-poor blood as its basic measure. It works by detecting changes in blood oxygenation and flow that occur in response to neural activity (BOLD signal). When a brain area is more active it consumes more oxygen and, to meet this increased demand, blood flow increases to the active area. Although the spatial resolution of fMRI is much worse than structural MRI, fMRI measures a signal that changes from moment to moment as neural activity waxes and wanes and so it provides an indirect measurement of neural activity over time.
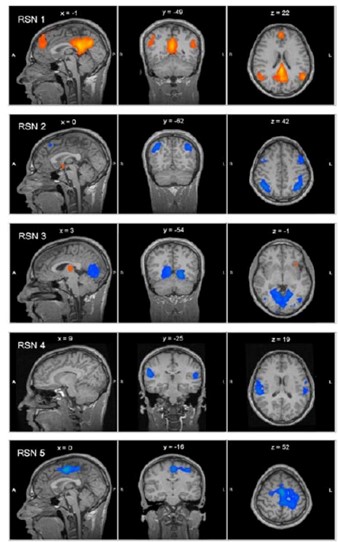
Resting-state fMRI does not require subjects to perform any specific task and evaluates the spontaneous neural activity in groups of brain areas that exhibit correlated BOLD signal time series, called resting state networks (RSN) (figure). There are several RSNs, including the default mode network (DMN) (RSN1), executive function network (RSN2), visual network (RSN3), auditory network (RSN4) and somatosensory network (RSN5). fMRI is becoming one of principal diagnostic method for brain in diseased or injured brain and represents a huge bulk of new research protocols for the mechanisms of brain functions. Moreover, the possibility to modulate brain functions with non-invasive brain stimulation techniques opens a new field for the research activity.
Noninvasive brain stimulation (NIBS)
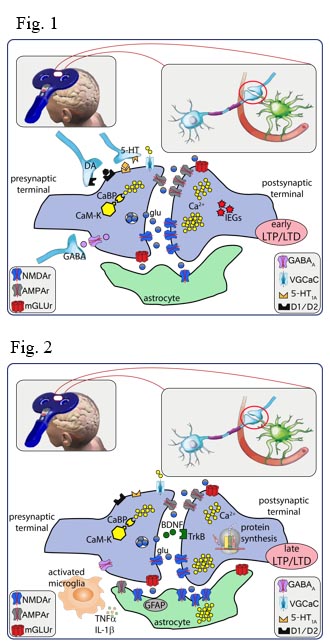
Noninvasive brain stimulation (NIBS) techniques have been proposed for the treatment of several neurological and psychiatric disorders. Their therapeutic effects have been related to bi-directional changes in cortical excitability depending on the applied techniques and stimulation protocol. Although after-effects are mostly short lived (Fig. 1), complex neurobiological mechanisms related to changes in synaptic excitability bear the potential to further induce therapy-relevant lasting changes (Fig. 2). Long-term potentiation (LTP) and depression (LTD) phenomena are insufficient in explaining the early and long-term changes taking place after short episodes of NIBS. Preliminary experimental studies indicate a complex scenario potentially relevant to the therapeutic effects, including gene activation/regulation, de novo protein expression, morphological changes, changes in intrinsic firing properties and modified network properties resulting from changed inhibition, homeostatic processes and glial function. Among NIBS techniques, repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS) have been found to be a promising noninvasive treatment for a variety of neurological and neuropsychiatric disorders.
 Behavioral tests
Behavioral tests
- Open field test evaluates exploratory behavior through analysis of grid crossing
- Rotarod test evaluates coordination and motor ability skills on the rotarod apparatus
- Footprint test evaluates gait analysis parameters
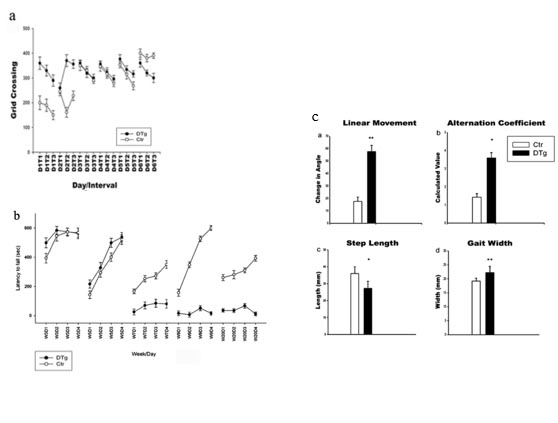
a) Open-field test, grid crossing analysis in control and ataxic mice
b) Rotarod test, increased latency to fall from the rotarod in ataxic mice
c) Footprint test, alteration of step parameters in ataxic mice
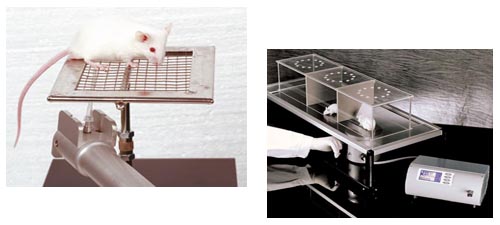
Behavioral tests
Von frey filament test: tactile allodynia
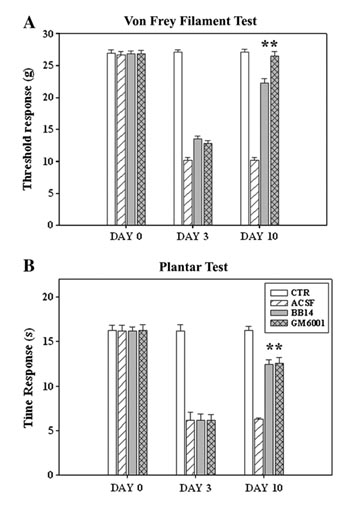 Plantar test: thermal hyperalgesia
Plantar test: thermal hyperalgesia
Antinociceptive effect of i.t. administration of NGF-like peptide (BB14) and GM6001, a metalloprotease inhibitor, in sciatic spared nerve injured (SNI) animals. SNI animals show a significant reduction of nociceptive thresholds (day 3 after SNI) and a significant improvement after intrathecal treatments for 7 days (day 10).